A Bard Powerport lawsuit is an individual lawsuit which is consolidated into the Federal MDL. Many victims are wondering, what is a powerport? The Bard PowerPort is a port catheter device, which is allegedly defective, leading to Bard Power Port lawsuits across the United States. In 2000 the FDA green-lighted the Bard powerPort. The medical device is a catheter implanted beneath the skin to supply extended, for a long duration, access to connect. This connection is without much difficulty, to supply medication and fluids that are intravenous, as well as supplying blood products. There have been numerous problems with the Bard Powerport design. The powerport catheter problems have lead to allegations of severe injuries as well as deaths. The catheter port material has been known to crack, leading the catheter to potentially fracture or even to migrate. These defects have caused grave insidious infections, blood clots, cardiac punctures and numerous life altering medical conditions and lead to an increase in Bard power port lawsuits. Power port claims have been pursued by victims who were hospitalized when their Bard Powerport device cracked, broke, leaked medication, or ceased working- necessitating an invasive surgery. Many of these victims are now pursuing an individual Bard Powerport lawsuit. The Bard Powerport lawsuits filed in U.S. Federal Courts have been consolidated in an mdl entitled: “In re: Bard Implanted Port Catheter Products Liability Litigation, MDL 3081, Case No. 2:23-md-03081-DGC (Hon. David G. Campbell).” This post also provides a Bard Powerport lawsuit update from September 2025
Bard Powerport Lawsuit
Victims are alleging that the Bard Power port is defectively designed. Victims are currently filing Bard Power Port lawsuits, alleging that Bard had knowledge that the catheters were likely to put victims at severe risk. Damages could be won as a result of a Bard catheter lawsuit for victims who were implanted with a Power port and endured one of the below complications:
- Infections
- Septic shock
- Hemmoraging injuries
- Sepsis
- Deep Vein Thrombosis (DVT)
- Bleeding Injuries
- Cardiac/pericardial tamponade
- Cardiac arrhythmia
- Severe and persistent pain
- Perforations of vessels, tissues as well as organs
- Death
- Assorted other injuries linked to the fractured Powerport catheter
Bard Powerport lawsuit Updates, July 2024
July 6th, 2024- The Bard Power Port MDL grew significantly by nearly 30% in June of 2024, from a bit over 230 lawsuits to nearly 300 claims. This lawsuit is not progressing as fast as some prognosticators believed.
March 10, 2024: Over 6 Bard PowerPort lawsuits were moved from federal Courts into the MDL in February 2024. There are over 106 lawsuits in the Bard Powerport class action mdl.
March 1, 2024: After a conference Judge Campbell set forth a case management order determining a number of issues argued at the conference. Justice Campbell refused to delay the proceedings per the manufacturer’s request. The Court ordered that bellwether trial selections should proceed.
February 23, 2024: Lawyers have filed two suggestions of death notices in February 2024 in the MDL. This shows the sad reality of the Powerport litigation. A “suggestion of death” in a lawsuit is a legal notice to the federal tribunal which establishes that a litigant in the litigation is dead. This legal maneuver is important because the passing away of a litigant may change the course and handling of the litigation. Bard Powerport lawyers may be required to amend the complaint, therebye, inserting the executor or administrator of the estate as the Plaintiff. The class action attorneys may proffer new allegations in the amended complaint for wrongful death and perhaps a cause of action for a survival claim.
February 19, 2024: There will be a status conference in the Bard Power Port lawsuit on March 1st, 2024.
February 15, 2024: The JPML federal justices panel ruled that the Bard Power Port lawsuits MDL (often described interchangeably as the “Bard Power Port Class action”) shall include lawsuits asserting that problems from the Bard port reservoir lead to infections. This determination significantly expanded the scope of the Bard litigation that in the past was limited to catheter lawsuits relating, solely, to defect issues with the catheter.
February 10, 2024: Over 25 new claims were filed into the Bard PowerPort class action MDL for the month of January 2024. This is the most new complaints filed into the the MDL since the inception of the consolidated Powerport lawsuits. There are now nearly 100 power port lawsuits in the MDL.
January 16, 2024: In the 2023 calender year only 69 lawsuits were filed into the federal MDL. It does not seem like this will ever be a huge MDL but only time will tell.
January 14, 2024: The JPML Panel shall convene in January 2024 to decide whether the Bard PowerPort MDL should be expanded. The vast majority of Power Port lawsuits assert a defect in the Bard PowerPort related to barium sulfate contained in the catheter. Victims allege that the catheter degrades as time passes, leading to possible catastrophic fractures. The MDL may now expand to lawsuits concerning allegations of defective port reservoirs.
January 1, 2024: The MDL Justice approved more time for the submission of a joint order concerning evidence preservation. Judge Campbell scheduled a conference on January 8, 2024.
December 20, 2023: This MDL (class action) is proceeding slowly as less than 10 new lawsuits were filed in the MDL class action in prior approximately 30 days. There are under 70 cases in the MDL. During the 5 month span since the MDL commenced, there have been under 20 new Bard Powerport lawsuits filed.
December 1, 2023: Justice Campbell issued a cohort of 6 new Case Management Orders (CMO 6-12). These orders establish the rules as well as procedures, explaining how the litigation will proceed in the near future. The case management orders set forth the plan of how the lawsuits will proceed through the document production process known as discovery. The CMO’s delineated how bellwether trials will be selected. Judge Campbell’s comprehensive orders pertained to establishing a master complaint as well whether short form complaints will be utilized in the litigation. The Justice addressed direct filing into the Bard MDL. It appears that Justice Campbell is attempting to streamline the Powerport litigation. Bard Power Port lawsuits can now be filed right into the MDL. It is no longer necessary for victims’ lawyers to pursue claims in different district courts across the United States!
There is now a master complaint filed into the litigation. This enables Bard Powerpoint attorneys to file Short-Form Complaints into the MDL litigation. The short form will allow victims to set forth the Bard Power Port model implanted in them as well as their specific injuries.
November 22, 2023: 2 new lawsuits were filed into to the Bard PowerPort class action MDL in the prior 30 days. At this time, there is under 65 cases in the MDL class action. It is expected that new claims will increase once case management orders go into affect and their is a short form complaint and a streamlined process.
November 21, 2023: Justice Campbell is pushing the Bard PowerPort causes of action towards forward progress.
November 16, 2023- The presiding justice, the honorable justice Campbell, held a case status conference in order to address progress made in the lawsuits and plans for future case management.
November 12, 2023- There are well over 60 PowerPort claims in the MDL in Federal Court in the U.S. District for the District of Arizona. It looks like there will also be a consolidated state Court litigation in New Jersey. This consolidate case in New Jersey is state Court MCL. (multi-county litigation). A number of Bard Power Port victims with filed lawsuits in New Jersey State Courts have asked the New Jersey Supreme Court to commence an MCL for Bard Powerport lawsuits. The victims assert that there will be over 500 lawsuits filed in the MCL, if it is established.
November 9, 2023- The parties set forth a joint memorandum (PDF), explaining issues to be addressed to the Justice at conference. The memo includes proposals / plans for bellwether trials in the PowerPort lawsuits. Bard Powerport lawyers drafted a joint memorandum. This joint memo set forth the issues in the case as well as a proposed plan for how bellwether trials would proceed. The proposed plan is that Plaintiff’s and Defendants would propose 24 cases, each, prior to July 1, 2024. After exchange of discovery, 15 lawsuits will be selected prior to December 17, 2024. Both Plaintiff and defendant lawyers will choose five lawsuits. The other 5 selections will be joint selections by the parties.
September 19, 2023 Judge Campbell in the Federal Bard Powerport class action MDL ratified the leadership structure proposed by lawyers in a case management order
September 5, 2023- Victims attorneys in the Federal MDL filed a proposal in the MDL. The attorneys asked for three lawyers to act as plaintiffs’ co-lead counsel, 11 lawyers to perform duties on a Plaintiffs’ Executive Committee (PEC), 13 attorneys serving on Plaintiffs’ Steering Committee (PSC) as well as 12 lawyers to be involved on numerous sub-committees. Two PEC lawyers will also be liason counsel.
September 1, 2023- DENE MAGNON a Powerport victim and a Lousiana resident, filed a Bard Power Port lawsuit IN THE UNITED STATES DISTRICT COURT DISTRICT OF NEW JERSEY against BECTON, DICKINSON AND COMPANY, C.R. BARD, INC. and BARD ACCESS SYSTEMS, INC.,
August 8, 2023- After the lawyers engaged in oral arguments, the JPML released a transfer order (PDF), relocating all Bard Port lawsuits filed in Federal Court to the U.S. District for the District of Arizona. The lawsuits will be handled by Justice David G. Campbell. Judge Campbell is well known to Bard because he presided over thousands of IVC filter lawsuits against Bard. The JPML reasoned that their are already 50 Bard Powerport lawsuits in U.S. Federal Courts filed in 28 assorted Federal Court districts.
6/9/2023: Lawyers representing Bard Power Port lawsuit victims filed a motion before the Judicial Panel on Multidistrict Litigation to consolidate all pending Bard Powerport lawsuits in the United States federal courts into an MDL. An MDL is not a Bard Power Port class action. Pursuant to the consolidation motion, at this time there are ten pending Powerport lawsuits in different federal courts. The motion filed by the victims indicates that there is a likelihood of many Bard power port lawsuits in the near future.
Injuries Caused by the Dangerous Powerport Device
The alleged defective design of the Bard Powerport makes the implanted device likely to fracture after the device is implanted. If the device fractures, it can migrate causing vascular damage. The Powerport can malfunction in a number of different ways.
Migration & fracture of the device can lead to a number of severe injuries which include:
- hemorrhage;
- cardiac arrhythmia
- death
- cardiac tamponade
- thromboembolism;
- infection;
- serious and consistent pain;
- life threatening perforations of vessels, tissues and human organs,
- more surgeries to eliminate the dangerous device.
In the world of healthcare, medical devices serve as integral components, facilitating numerous treatments and patient care routines. However, these devices may occasionally malfunction or cause unforeseen harm, leading to significant legal implications.
One such case is the Bard Powerport Lawsuit, a legal dispute centered around allegations of defects in the Powerport devices manufactured by C.R. Bard. These devices, pivotal in delivering long-term medical treatments such as chemotherapy and total parenteral nutrition (TPN), have been implicated in severe health complications arising from device failures. This sparked the lawsuit, bringing more profound issues about medical device safety and corporate accountability to the fore.
Understanding the Bard PowerPort Device
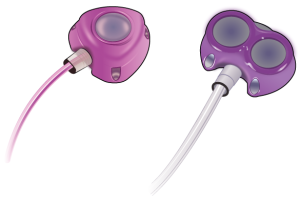
In the realm of medical technology, the Bard Power Port device emerged as a game changer, offering an implantable venous access system designed to streamline long-term intravenous (IV) therapies. This device provides a centralized access point to the patient’s bloodstream, thereby considerably reducing the need for repeated venous punctures. This process often proves to be uncomfortable and stressful for patients, particularly those undergoing extended treatment periods. The Powerport device comprises a small, circular reservoir connected to a catheter. This reservoir is surgically implanted beneath the skin, typically in the upper chest area, while the catheter is inserted into a large vein leading directly to the heart.
Powerport catheter
When correctly implemented and operational, this system enables efficient and less painful administration of medications or nutrients directly into the bloodstream— significantly improving the quality of life for patients requiring consistent Intravenous therapy. However, the Powerport device, like any medical implement, is not devoid of potential risks. These could be associated with the implantation procedure or the device’s subsequent performance within the body. Manufacturers like Bard, therefore, bear a considerable responsibility to ensure the safety and effectiveness of their devices, from the stages of design and manufacture through to post- implantation usage. This backdrop sets the stage for the reported failures and ensuing lawsuits associated with the Bard Powerport device.
The Bard Powerport™ ClearVUE™ Implantable Port is a port/catheter system manufactured by Bard. According to the manufacturer, “the PowerPort is a totally implantable vascular access Device designed to provide repeated access to the vascular system for the delivery of medications, intravenous fluids, parenteral nutrition solutions, and blood products. The intended purpose of the PowerPort is to make it easier to deliver medications directly into the patient’s bloodstream. The Device is surgically placed completely under the skin and left implanted. The PowerPort is a system consisting of two primary components: an injection port and a polyurethane catheter.” Dene Magnon complaint
Reports of Powerport Failures
Despite its initial promise and utility, the Bard PowerPort has been associated with various device failures. Patients implanted with the PowerPort have reported various complications, ranging from infections and clotting to more severe problems like device fracture and migration. Device fractures pose a particularly significant risk. This failure mode involves the catheter component of the PowerPort breaking apart, with fragments potentially traveling through the patient’s bloodstream. These fragments can lead to severe and often life-threatening conditions. One such condition is a pulmonary embolism, a blockage in one of the pulmonary arteries in the lungs. This condition often requires immediate medical intervention and can prove fatal if not promptly treated.
The issue of device migration is another grave complication. The PowerPort device moves from its original placement site within the patient’s body. This movement can cause significant internal damage, especially if the device impinges upon vital organs or tissues. Such complications could result in severe internal bleeding, damage to critical organs, or serious infection. Moreover, the device could lose effectiveness or become entirely non-functional if it migrates, disrupting the patient’s treatment schedule.
Accusations and Allegations
As the number of reported PowerPort failures and their associated severe health complications mounted, a series of lawsuits were filed against C.R. Bard. The plaintiffs in these lawsuits alleged that Bard was fully aware of the potential defects and failures of their PowerPort devices. Despite this knowledge, the company allegedly needed to provide adequate warnings to healthcare providers and patients about the associated risks. The accusations leveled against C.R. Bard extended beyond simple negligence. Plaintiffs charged the company with fraudulent concealment, alleging that Bard knowingly withheld information about product defects. They also accused the company of breaching warranty, arguing that Bard failed to live up to their product safety and effectiveness promise.
These lawsuits targeting Bard’s PowerPort device are part of a broader controversy surrounding C.R. Bard and its range of medical devices. The company has faced legal challenges regarding other products, such as its transvaginal mesh, hernia mesh and IVC filters. These devices, like the PowerPort, were alleged to have serious defects that led to complications and adverse health outcomes for patients.
Legal Proceedings and Settlements
The legal landscape concerning the Bard PowerPort lawsuits is rife with complexities. It has been the backdrop for a series of protracted battles, some of which have culminated in settlements, while others continue to evolve within the courtrooms. One of the primary objectives of the legal proceedings is to provide redress to the plaintiffs. These are patients who, in many cases, have experienced significant physical and emotional distress due to complications arising from the Bard PowerPort device. Lawsuits commonly seek damages to compensate for various factors such as medical expenses, pain and suffering, and loss of earnings caused by the health complications resulting from PowerPort device failures.
PowerPort wrongful death lawsuits
When settlements have been reached, they generally follow exhaustive negotiations between the plaintiffs and Bard. The amounts involved in these settlements vary, primarily determined by the severity of the plaintiff’s injuries, the extent of their medical expenses, and the impact the complications have had on their quality of life. Furthermore, settlements often incorporate non-economic factors such as pain, suffering, and loss of enjoyment of life, which can significantly increase the compensation the plaintiffs receive.
However, settling can be challenging. In some cases, Bard has defended its position vigorously in court. These ongoing litigations can be lengthy and often involve presenting complex medical evidence, expert testimonies, and extensive legal arguments. The uncertainty inherent in these proceedings means that the final verdicts and potential compensation amounts can vary substantially from case to case.
Bard Powerport class action lawsuit?
Thankfully there are no Bard Powerport class action suits in the United States. Power Port class actions only benefit the trial lawyers and the victims end up with peanuts. Avoid a Power port class action suit at all costs.
Bard Power port catheter Lawsuits serve as a stern reminder
At the same time, these lawsuits serve as a stern reminder of medical device manufacturers’ responsibilities to their customers. Each case brings into focus the fundamental principle that companies like Bard are expected to uphold: the safety and well-being of patients. The lawsuits have cast a harsh light on the company’s practices, and the large or small settlements act as a form of accountability, underlining the necessity for stringent manufacturing standards and full disclosure
of potential device risks.
Power port for chemo
Moreover, the settlements and ongoing litigations provide invaluable lessons forthe wider medical device industry. They underscore the high stakes in medical device manufacturing and emphasize the need for robust quality control mechanisms, effective patient communication, and swift action when device defects become apparent. This heightened awareness could prompt industry-wide changes, encouraging manufacturers to prioritize patient safety.
In-depth Investigation and Expert Testimonies
As part of the legal proceedings, detailed investigations are often carried out into the design and manufacturing processes of the PowerPort device. These investigations also examine Bard’s knowledge and handling of the potential defects. Expert medical testimonies often play a crucial role in supporting the plaintiffs’ claims. These testimonies involve an intricate analysis of the device’s impact on patient health and the severe complications resulting from device
failures.
However, it’s crucial to note that each case is unique. The severity of complications and the extent of health impacts can vary significantly among patients. Therefore, compensation amounts can vary, often reflecting the severity of health impacts and other factors.
The Continuing Legal Battle and Future Implications
While many lawsuits involving Bard’s PowerPort have been settled, providing relief to the affected patients, several legal battles remain ongoing. These pending cases serve as a stark reminder of the ongoing debates surrounding medical device safety and the responsibilities of manufacturers to ensure patient safety. The outcomes of these lawsuits could carry significant implications for the future of the medical device industry. If manufacturers are held accountable for device failures, it could lead to enhanced scrutiny of product design and manufacturing processes.
Additionally, health authorities may respond by implementing more stringent regulations. In the long run, these changes could lead to the production of safer medical devices and an overall improvement in patient care. Looking Forward: Medical Device Safety and Corporate Accountability As the Bard PowerPort lawsuits unfold, they highlight the complex interplay between medical device safety, corporate accountability, and patient welfare. The outcomes of these legal disputes could prompt changes in how medical device manufacturers approach product safety.
Potential for improved regulatory oversight
The cases also highlight the potential for improved regulatory oversight by health authorities to ensure patient safety. The ripple effects of these lawsuits could be far-reaching. The medical device industry may be compelled to reassess its design and manufacturing protocols. Healthcare providers may become more vigilant in selecting medical devices for patient care. Lastly, patients may become more informed and proactive in understanding the potential risks associated with their treatments.
bard power port
The Bard PowerPort Lawsuit is critical in discussing medical device safety and corporate responsibility. As these legal proceedings continue, they underscore the potential risks associated with medical devices and manufacturers’ essential role in ensuring patient safety. The outcomes of these catheter migration lawsuits may shape industry practices and regulatory standards for years to come, leading to safer and more effective medical devices in the future. Bard victims should check this page frequently for a Bard Powerport lawsuit update. We also represent clients in ozempic lawsuits, asbestos trust fund lawsuits and hair relaxer lawsuits
References
1. Krouse, L. (2023). Bard PowerPort Lawsuit. Lawsuit Information Center. Retrieved
May 30, 2023, from
2. Staff Writer. (2023). Bard PowerPort Lawsuit. AboutLawsuits.com. Retrieved May
30, 2023, from https://www.aboutlawsuits.com/bard-powerport-lawsuit/
3. FDA. (2018). MAUDE Adverse Event Report: C.R. Bard Peripheral Vascular
PowerPort Implantable Port. FDA. Retrieved May 30, 2023, from
4. Smekens, N., Van Looy, S., & Droissart, M. (2011). Fracture and migration in the
pulmonary artery of a totally implantable venous access device. Journal of Vascular
Access. DOI: 10.5301/jva.2011.6286
5. C.R. Bard, Inc. (2021). Bard Access Systems PowerPort* Implantable Port.
Retrieved May 30, 2023, from
